Putting Science to Work for the Health of Women
For more than 30 years, the Office of Research on Women’s Health (ORWH) has served as the focal point for women’s health research at NIH. Situated within the NIH Office of the Director, ORWH works in partnership with the other NIH Institutes, Centers, and Offices (ICOs) to promote the prioritization of women’s health across research portfolios, the inclusion of women in research populations, and the consideration of sex and gender as critical factors in health and disease. Since its inception, ORWH also has developed innovative strategies to recruit, retain, and support women in the biomedical workforce.
ORWH leads a diverse portfolio of research programs, policy and planning initiatives, events, and resource development aimed to stimulate and advance research that improves the health of women from head to toe and across the lifespan, positively impacting the health of all.
Picture a world in which the biomedical research enterprise thoroughly integrates sex and gender influences; every woman receives evidence-based disease prevention and treatment tailored to her own needs, circumstances, and goals; and women in science careers reach their full potential.
NIH Associate Director for Research on Women's Health Director, NIH Office of Research on Women's Health
Established in 1990, ORWH became the first Public Health Service office dedicated specifically to promoting women’s health. It was established by then–NIH Acting Director of the National Institutes of Health (NIH) William F. Raub, Ph.D., in response to congressional, scientific, and advocacy concerns about the inconsistent and infrequent inclusion of women in NIH-supported clinical research and the lack of research on women’s health. This widespread lack of inclusion of females in clinical trials and resulting evidence gap meant that many clinical decisions about female health care were based on findings from studies of male participants—without evidence that these findings were generalizable. In 1993, Congress affirmed the establishment of ORWH in statute by the NIH Revitalization Act of 1993 (Public Law No. 103-43, Section 486), assigning the office a far-reaching mandate, including:
- Advise the NIH director and staff on matters relating to research on women’s health
- Strengthen and enhance research related to diseases, disorders, and conditions that affect women
- Ensure that research conducted and supported by NIH adequately addresses issues regarding women’s health
- Ensure that women are appropriately represented in biomedical and bio-behavioral research studies supported by NIH
- Develop opportunities and support for recruitment, retention, reentry, and advancement of women in biomedical careers
- Support and advance rigorous research that is relevant to the health of women
- Ensure NIH-funded research accounts for sex as a biological variable (SABV)
NIH Revitalization Act
In addition to confirming ORWH in statute, the NIH Revitalization Act of 1993 created the NIH Advisory Committee on Research on Women’s Health (ACRWH) and the Coordinating Committee on Research on Women’s Health (CCRWH) that together advise the ORWH director on topics related to women’s health research. The NIH ACRWH consists of leading non-federal experts in many fields and provides the ORWH director with recommendations from an external perspective. The NIH CCRWH consists of NIH ICO directors or their designees who can offer suggestions based on internal knowledge of NIH and its processes. This robust advisory structure ensures that ORWH considers an external, non-federal perspective, as well as an NIH-wide perspective.
With the passage of the NIH Revitalization Act in 1993, Congress also enshrined into law the policy of inclusion of women and minorities in NIH-supported clinical research, which ORWH plays a key role in enforcing and refining. In 1994 NIH revised its inclusion policy to comply with the statutory language. The NIH Revitalization Act essentially reinforced certain existing NIH policies, stating that NIH should ensure that:
- Women and minorities and their subpopulations are included in all clinical research
- Women and minorities and their subpopulations are included in Phase III clinical trials in a way that enables performance of valid analyses
- Cost is not allowed as an acceptable reason for excluding these groups
- It initiates programs and support for outreach efforts to recruit and retain women and minorities and their subpopulations as participants in clinical studies
NIH Reform Act
The NIH Reform Act of 2006 (Public Law No. 109-482) paved the way for ORWH to become part of the Division of Program Coordination, Planning, and Strategic Initiatives (DPCPSI) in the NIH Office of the Director. DPCPSI coordinates NIH-wide initiatives and identifies emerging scientific opportunities, rising public health challenges, and scientific knowledge gaps that merit further research. ORWH’s location within DPCPSI well positions it to coordinate across the NIH ICOs to highlight research needs, stimulate and support collaborative efforts, and set the agenda for new research on the health of women.
21st Century Cures Act
In 2016, Congress passed the 21st Century Cures Act (Public Law No. 114-255), which introduced many significant changes associated with ORWH’s role in the promotion of the health of women across NIH. First, the act requires that CCRWH members, who serve as liaisons between ORWH and the ICOs, be either IC directors or their senior-level staff designees. The act also requires the IC directors to consult annually with the ORWH director about their objectives to ensure that they are addressing women’s health needs and are focused on reducing women’s health disparities. Lastly, the strategic plans issued by the individual ICs, required at least every 6 years, must document these same priorities.
Inclusion Across the Lifespan
Another major policy set forth in the 21st Century Cures Act is the Inclusion Across the Lifespan policy, which applies to all grant applications and contract solicitations submitted on or after January 25, 2019. This policy has expanded previous policies for the inclusion of women, minorities, and children in clinical research to include individuals of all ages; states that justifications for exclusion criteria based on age must have valid ethical or scientific reasons; and requires the provision of participants’ ages at enrollment in progress reports. The 21st Century Cures Act also requires that applicable Phase III clinical trials report their results in ClinicalTrials.gov by sex/gender and by race and ethnicity.
Sex as a Biological Variable
NIH and ORWH implemented the Policy on Sex as a Biological Variable (SABV) in 2016. The SABV policy requires applicants for NIH grants for vertebrate animal and human studies to explain their plans to factor sex as a biological variable into their studies or to provide scientific justification for a single-sex study.
The implementation of the Inclusion and SABV policies serves as a major milestone in achieving the goals of ORWH, because they ensure that women, people of all ages, and members of underserved racial and ethnic groups are appropriately represented in clinical research and that the treatments identified will be effective for these populations.
Consolidated Appropriations Acts
As part of the Consolidated Appropriations Acts, 2023 (Public Law 117-328) Congress directed NIH to establish an Office of Autoimmune Disease Research (OADR-ORWH) in ORWH to amplify advancement of research for these chronic conditions, with approximately 80% of those affected being women.
Explore our history: Graphic Timeline - 30 Years of Advancing Women's Health
ORWH’s mission is multifold:
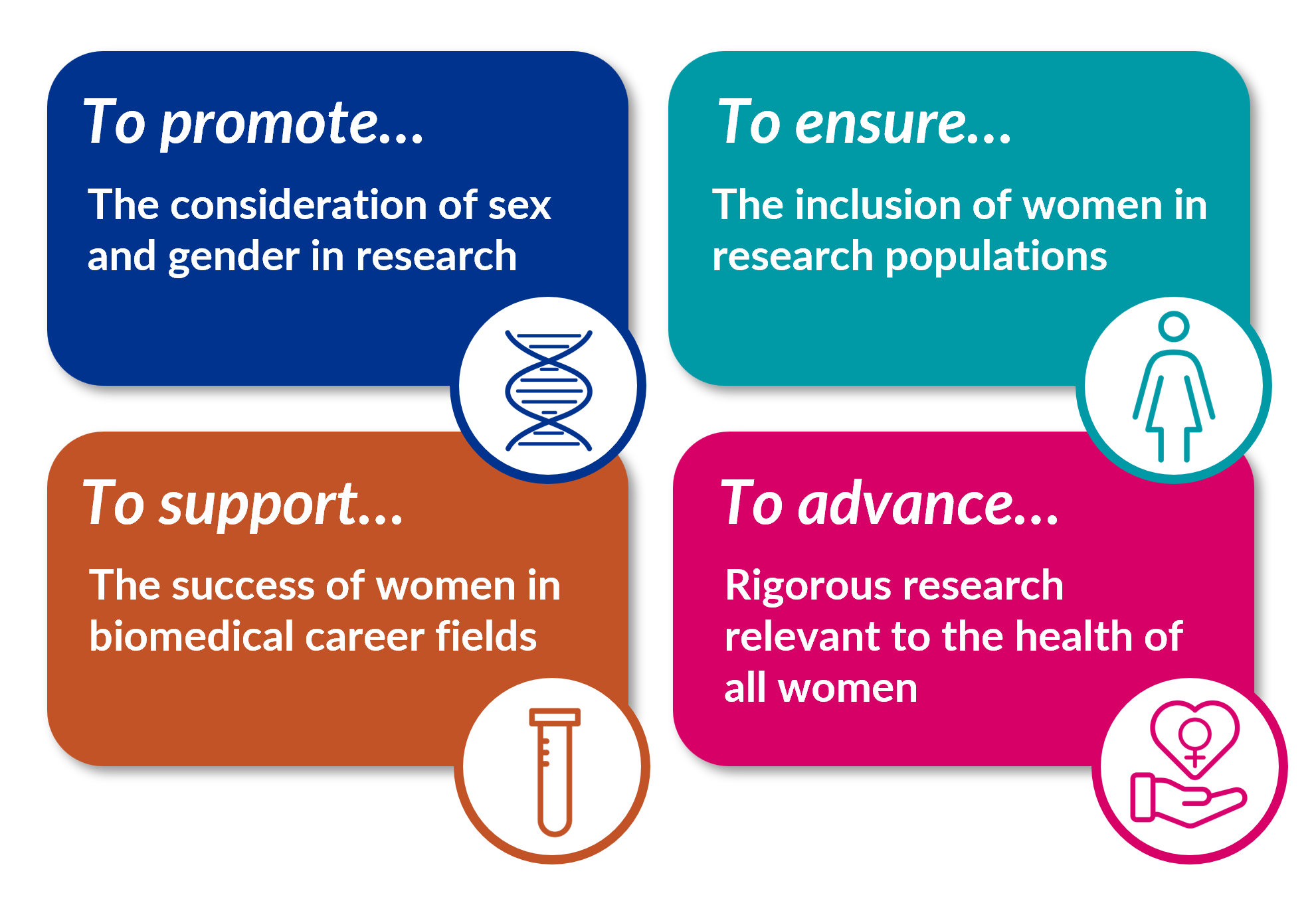
Together with NIH, ORWH envisions a world in which…
All womeni receive evidence-based disease prevention and treatment tailored to their own unique needs, circumstances, and goals;
Women, people of all ages, and underrepresented racial and ethnic groups are appropriately represented in clinical research populations;
Sex and gender are considered at every stage of biomedical research and health care delivery; and
All women in scientific career fields are empowered to reach their full potential.
Each aspect of this vision is deeply interconnected with the others. In order for women to receive medical care tailored to their needs, clinicians must have access to and awareness of scientific data that describe the influences of sex and gender in various diseases and conditions, such as sex-based differences in symptom presentation or treatment response. To discover and disseminate information from these data, women must be included in research and researchers must intentionally consider sex and gender as factors in their research design, analysis, and reporting of biomedical studies. In addition, greater research focus is needed about women’s health issues such as endometriosis, menopause, and menstrual disorders to improve their diagnostics and clinical treatment options. Because workforce studies indicate that women scientists are more likely than their male counterparts to conduct research in these areas, supporting women’s careers in the biomedical workforce is critical for advancing research on the health of women.
Learn More about our Mission Areas:
- Sex and Gender Influences in Health and Disease
- Women’s Health Equity and Inclusion
- Supporting Women in Biomedical Careers
i ORWH uses the term “women” as inclusive of all people who identify as women. ORWH is committed to advancing research relevant to the health of all women and all people assigned female at birth.
Despite great progress and advancements in women’s health research, much work remains to be done to achieve a vision of women’s health equity. Women continue to be disproportionately affected by many diseases and conditions, such as autoimmune diseases and chronic pain. Women are also differently affected by many diseases and conditions, presenting different symptoms and responding differently to treatments and thereby underscoring the criticality of considering sex as a biological variable in all forms of health research and treatment development. Furthermore, female-specific conditions such as endometriosis and polycystic ovary syndrome continue to be understudied areas despite significant disease burden. ORWH remains dedicated to identifying research gaps and galvanizing efforts to address them. Recent areas of focus include maternal health, women’s mid-life health and menopause research, HIV, and the burden of chronic disease among women, particularly that of autoimmune diseases (see OADR-ORWH). Learn more about current NIH priorities and efforts related to women’s health in the NIH-Wide Strategic Plan for Research on the Health of Women 2024-2028.

